Dr. Wesley Gray's Current Research Interests
![]()
![]()
![]()
![]()
"The Molecular Endocrinology Research Group" (MERG) here at SUBR is houses in the Chemistry and Environmental Toxicology departments, located in the Lee Hall and the Health Research Center. MERG is helping to pioneering a new field of research known as Nutrigenomics aim at understanding how bioactive chemical from medicinal plants influence health and disease. Natural products and common over the count herbal supplement produces a multitude of effects including lowering incident of breast and prostate cancer, reduce the risk of cardiovascular disease and decrease the risk of osteoporosis. The major research activities in our laboratory, focuses on understanding how hormonal compounds present in medicinal plants, natural products and phytochemicals in everyday foods protect again breast and prostate cancer. Our major research activities focus on steroid hormones and cancer with an emphasis on plant estrogenic/androgenic compounds that modulate hormone-dependent processes. MERG focuses on: (i) isolating and characterization of genes (biomarkers) that may be used to study the biological function of dietary estrogens commonly referred to as phytoestrogens; (ii) we are applying chemoinfomatic, protenomics, enzymology, chemistry and molecular biology in deciphering the cellular mechanism of action of phytoestrogen at the level of the gene and protein; (iii) We are using cell and animal models to understanding how phytochemical hormones modulating the expression of developmentally regulated genes in the male reproductive system and (iv) Appling one and two-dimensional separation techniques of chemical profiling of medicinal active natural extract for bioactive compounds
Medicinal Plants and Cancer: A study of Bizzy Nut as a cancer preventative Agent in ProstateCancer
Currently, there is a shortage in prostate cancer preventives and therapeutics. The potentially curative standard therapies for PCa are limited to radical prostatectomy or irradiation, both of which are effective for localized disease. Furthermore, androgen ablation therapy can reduce the systemic tumor burden, but it is ineffective in treating androgen refractory tumors. Thus, studding Biz-2, a novel extract of tea that impairs growth of both androgen-dependent and androgen-independent prostate cancer cells, as a source for identifying new agents that can prevent and/or treat prostate cancer are of paramount importance. Our approach of using a combination of chemoinformatics and bioinformatics concurrently to explore the number of potential compounds present in Biz-2 and the pathways that they affect using three in vitro prostate cancer models in highly innovative. Thus, the overall objective of this proposal, namely demonstrating that the Kola acuminate (Bizzy Nut) extract contains bioactive compounds capable of functioning as chemo preventative agents against prostate cancer. The initial focus of this proposal is to isolate and identify the bioactive compounds found in the Biz-2Fr.3 fraction of Bizzy Nut and demonstrate, in vitro, its anti-cancer/chemopreventative potential using three prostate cancer cell models. To this end, two specific aims were developed.
Aim 1: To isolate and characterize the bioactive compounds in Bizzy nut extract associated with its anti-cancer properties in prostate cells. The goals of this Aim are to (1) Isolate and characterize the bioactive components of Biz-2 by preparative HPLC; (2) elucidate the structure of the compounds in Biz-2Fr.3 using a combination of UV-Vis, FTIR, NMR spectroscopy, LC-MS; and (3) Demonstrate the anti-cancer capability of the bioactive substances in Biz-2Fr.3.
Aim 2: To Identify the Biz-2Fr.3-induced apoptotic signaling pathways and determine the molecular mechanisms by which Biz-2FR.3 induces apoptosis in prostate cells. The goals of this Aim is to; (1) determine the mechanism of Biz-2Fr.3 anti-tumor activity using change in cell cycle distribution and protein expression profiling of cyclines. (2) Determine the involvement of mitochondria in Biz-2Fr.3 induction of apoptosis in normal and cancerous prostate cells using three prostate cell lines, the AR+ LNCaP, the AR- DU145 cells and RWP-1a normal transformed cell line.
**************************************************
Research Focus II
Phytoestrogenic Chemical and gene Expression
******************************************************
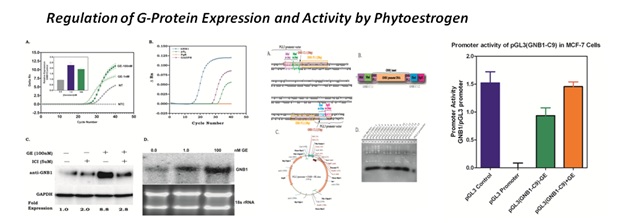 |
In mammalian systems, phytoestrogens (PEs) cause a multitude of effects including lower incidence of breast and prostate cancers, reducing the risk of cardiovascular disease, and decreasing the risk of osteoporosis in post-menopausal women (1-3). As a result of these beneficial actions, PEs have been given a great deal of attention in recent years. The effects of PE are thought to result from these compounds activating either the estrogen receptors (ER)-a or ER-p in target organs. Regulation of gene expression by the ER arises from the direct interaction of the receptor with ligand such as estradiol (E2) and interaction of the ligandreceptor complex with co-activators or co-repressors and components of the general transcription machinery (4,5). Modulation of ER-responsive genes by E2 is well known; however, the transcriptional activation of genes by PEs and other non-steroidal estrogens is poorly understood. Although studies have shown that certain PEs modulate estrogen action, the molecular mechanism of action of these compounds in cancer cells remains unclear. We previously identified a PE-responsive gene, GNB1, which is selectively activated by selective PEs and marginally by E2. GNB1 is a novel gene that has not previously been known to be regulated by PE and is dependent on the estrogen receptor for expression. Thus, this gene may serve as an ideal model to be use in understanding the role dietary estrogen plays in breast cancer cells at the molecular level. In this proposal, we will establish GNB1as a model system for studying PE-dependent gene expression by elucidating the mechanisms of the GNB1 gene's regulation. To accomplish this, we will characterize GNB1 regulatory elements (aim 1) with specific emphasis on, how PEs, in conjunction with ER lead to GNB1 transcriptional activation in cells (aim 2). To better understand the mechanism governing PE activation of GNB1, we will test the hypothesis that differs in GNB1 expression resulting from activity on the GNB1 upstream enhancer and interaction of these elements with a PE/ER complex. Thus, to investigate whether PEs are stimulating GNB1 expression at the level of transcription and which cis- and trans-acting transcriptional factors may mediate this stimulation, the following specific aims were developed.
Aim 1. Establish that transcriptional activation of GNB1 gene is achieved through the presence of a PE specific responsive element in the 5'- or 3'-fIan king region of the gene.
Aim 2. Characterize the regulatory elements present in the GNB1 gene that mediate phytoestrogen and estradiol transcription by developing and testing several GNB1 luciferase reporter gene constructs in MCF-7 breast cancer cells.
| RELATED INFO |
- Environmental Toxicology HomePage
- Admission Requirements
- Faculty and Staff
- Financial Assistance
- Graduate Curriculum
- Student Research Education Enhancement Program
- Announcements
